
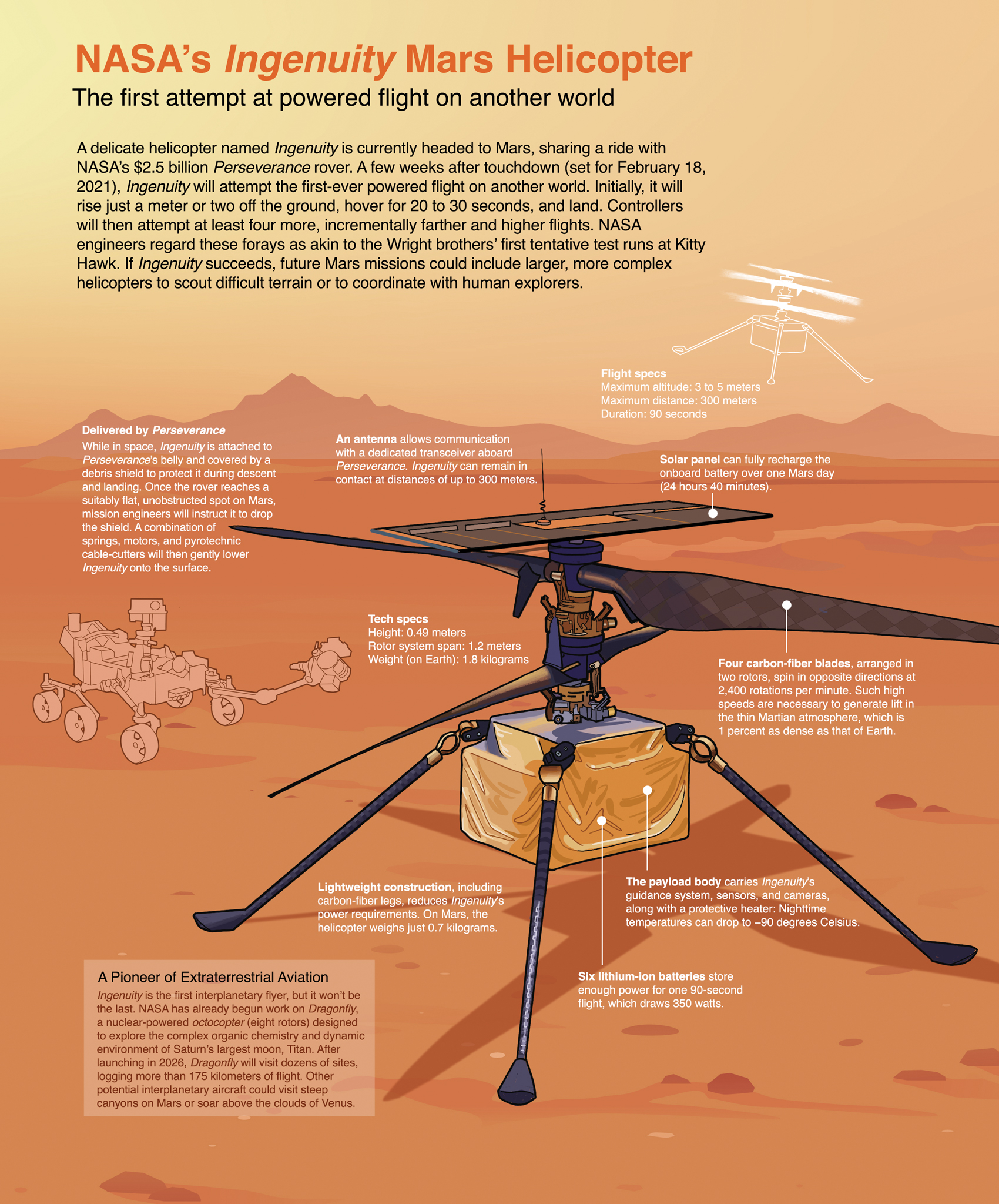
The ions move one way when the battery charges (when it's absorbing power) they move the opposite way when the battery How a lithium-ion battery charges and dischargesĪnimation: Charging and discharging a lithium-ion battery.Īs their name suggests, lithium-ion batteries are all about the movement of lithium ions: They prevent the overcharging and overheating thatĬan cause lithium-ion batteries to explode in some circumstances. Unlike simpler batteries, lithium-ion ones have built in Keep on discharging, at a very slow rate, even with the appliance disconnected). The battery essentially stops discharging at a high rate (but it does Whatever the battery is powering, the flow of electrons stops and so does the flow of ions. If ions stop moving through theĮlectrolyte because the battery completely discharges, electrons can't move through the outerĬircuit either-so you lose your power. The movement of ions (through the electrolyte) and electrons (around the external circuit, in the oppositeĭirection) are interconnected processes, and if either stops so does the other. It's effectively an insulating barrier, so far as electrons are concerned. Electrons do not flow through the electrolyte: In bothĬases, electrons flow in the opposite direction to the ions around Lithium ions move back across the electrolyte to the positiveĮlectrode, producing the energy that powers the battery. The battery takes in and storesĮnergy during this process. Negative, graphite electrode and remain there. Some of its lithium ions, which move through the electrolyte to the When theīattery is charging up, the lithium-cobalt oxide, positive electrode gives up Preferable to incinerating them or sending them to landfill.Īll lithium-ion batteries work in broadly the same way. Photo: Lithium-ion (Li-ion) batteries are less environmentally damaging thanīatteries containing heavy metals such as cadmium and mercury, but recycling them is still far

Too important in understanding the basic idea of how the battery works. The electrolyte varies from one type of battery to another-but isn't The negative electrode is generally made from carbon (graphite) and Newer batteries, from lithium iron phosphate (LiFePO 4). The positive electrode is typically madeįrom a chemical compound called lithium-cobalt oxide (LiCoO 2) or, in (connected to the negative or − terminal), and a chemical called anĮlectrolyte in between them. The battery's positive or + terminal), a negative electrode Has essentially three components: a positive electrode (connected to Of one or more power-generating compartments called cells.
Like any other battery, a rechargeable lithium-ion battery is made This battery is rated as 10.8 volts and has three cells inside. Produces 10–16 volts typically needs three to four cells. Each cell produces about 3–4 volts, so a lithium ion battery that Photo: A lithium-ion battery, such as this one from a laptop, is made from a number Often you use it and how well you look after it). Or three to as much as 10 years of useful life (depending on how So a rechargeable battery will typically give you anything from two TheseĬhemical reactions can happen hundreds of times in both directions,

Go in the opposite direction and the battery absorbs power. When the battery is discharging the reactions go one way and theīattery gives out power when the battery is charging, the reactions That the chemical reactions in a rechargeable battery are reversible: Split apart through entirely different reactions. Rechargeable batteries = reversible reactionsĭifferent chemicals are used in rechargeable batteries and they Happen only once and in only one direction: that's why ordinary batteries usuallyĬan't be recharged. The only trouble is, this chemical reaction can To which the battery's connected, providing electrical energy that drives theįlashlight. Ions move through the battery the electrons go through the circuit Themselves together to make other chemicals, producing a stream of positivelyĬharged particles called ions and negatively charged electrons. Connect the two ends of a battery to something like aįlashlight and chemical reactions begin: chemicals inside theīattery slowly but systematically break apart and join If you've read our main article on batteries, you'll know aīattery is essentially a chemical experiment happening in a small Once they're empty of electrical energy, there's no easy way to refill them. How a lithium-ion battery charges and dischargesĪrtwork: Ordinary batteries, such as zinc-carbon and alkaline ones, cannot be recharged because the chemical reactions that generate the power are not reversible.Rechargeable batteries = reversible reactions.


 0 kommentar(er)
0 kommentar(er)
